Naming binary compounds using stock system
Chemical nomenclature is far too big a topic to treat comprehensively, and it would be a useless diversion to attempt to do so in a beginning course; most chemistry students pick up chemical names and the rules governing them as they go along.
But we can hardly talk about chemistry without mentioning some chemical substances, all of which do have names— and often, more than one!
All we will try to do here is cover what you need to know to make sense of first-year chemistry. For those of you who plan to go on in chemistry, the really fun stuff comes later! There are more than million named chemical substances.
Who thinks up the names for all these chemicals? Are we in danger of running out of new names? The answer to the last question is "no", for the simple reason that the vast majority of the names are not "thought up"; there are elaborate rules for assigning names to chemical substances on the basis of their structures. These are called systematic names ; they may be a bit ponderous, but they uniquely identify a given substance.
PREPCHEM Writing Formulas and Naming Compounds
The rules for these names are defined by an international body. But in order to make indexing and identification easier, every known chemical substance has its own numeric "personal ID", known as a CAS registry number.
About 15, new numbers are issued every day. CAS registry numbers are essential tools for navigating through the forest of multiple names for a given substance.
Naming Covalent Molecular CompoundsFor example, ethanolCH 3 CH 2 OH, is also known as ethyl alcohol, grain alcohol, absolute alcohol, hydroxyethylene, and ethyl hydrate — but each bears the same registry number Many chemicals are so much a part of our life that we know them by their familiar names, just like our other friends. A given substance may have several common or trivial names ; ordinary cane sugar, for example, is more formally known as "sucrose", but asking for it at the dinner table by that name will likely be a conversation-stopper, and I won't even venture to predict the outcome if you try using its systematic name in the same context:.
But "sucrose" would be quite appropriate if you need to distinguish this particular sugar from the hundreds of other named sugars. The only place you would come across a systematic name like the rather unwieldy one mentioned here is when referring in print or in a computer data base to a sugar that has no common name.
Chemical substances have been a part the fabric of civilization and culture for thousands of years, and present-day chemistry retains a lot of this ancient baggage in the form of terms whose hidden cultural and historic connections add color and interest to the subject. Many common chemical names have reached us only after remarkably long journeys through time and place, as the following two examples illustrate:.
Most people can associate the name ammonia NH 3 with a gas having a pungent odor; the systematic name "nitrogen trihydride" which is rarely used will tell you its formula. What it will not tell you is that smoke from burning camel dung the staple fuel of North Africa condenses on cool surfaces to form a crystalline deposit.
The ancient Romans first noticed this on the walls and ceiling of the temple that the Egyptians had built to the Sun-god Amun in Thebes, and they named the material sal ammoniac, meaning "salt of Amun".
InJoseph Priestly the discoverer of oxygen found that heating sal ammoniac produced a gas with a pungent odor, which a T. Bergman named "ammonia" eight years later. Arabic alchemy has given us a number of chemical terms; for example, alcohol is believed to derive from Arabic al-khwl or al-ghawl whose original meaning was a metallic powder used to darken women's eyelids kohl.
Alcohol entered the English language in the 17th Century with the meaning of a "sublimated" substance, then became the "pure spirit" of anything, and only became associated with "spirit of wine" in Finally, init become a part of chemical nomenclature that denoted a class of organic compound. But it's still common practice to refer to the specific substance CH 3 CH 2 OH as "alcohol" rather then its systematic name ethanol.
The general practice among chemists is to use the more common chemical names whenever it is practical to do so, especially in spoken or informal written communication. For many of the very simplest compounds including most of those you will encounter in a first-year coursethe systematic and common names are the same, but where there is a difference and if the context permits it, the common name is usually preferred.
Many of the "common" names we refer to in this lesson are known and used mainly by the scientific community. Chemical substances that are employed in the home, the arts, or in industry have acquired traditional or "popular" names that are still in wide use.
Many, like sal ammoniac mentioned above, have fascinating stories to tell. Here is a brief sample of some other traditional names:. A more extensive list of common- and trade names can be found here. Minerals are solid materials that occur in the earth which are classified and named according to their compositions which often vary over a continuous range and the arrangement of the atoms in their crystal lattices.
Naming Ionic Compounds Using the Stock System Flashcards | Quizlet
There are about named minerals. Many are named after places, people, or properties, and most frequently end with - ite. See here for an extensive list. Chemistry is a major industry, so it is not surprising that many substances are sold under trademarked names.
This is especially common in the pharmaceutical industry, which uses computers to churn out names that they hope will distinguish a new product from those of its competitors. Perhaps the most famous of these is Aspirinwhose name was coined by the German company Bayer make money in playstation home This trade name was seized by the U.
Some interesting names Those who don't think that chemists have a sense of humor should have a look at this site by Prof. Paul May of the University of Bristol in the UK:.
Molecules with Silly or Unusual Names. Naming of chemical substances begins with the names of the elements. The discoverer of an element has traditionally had the right to name it, and one can find some interesting human and cultural history in these names, many of which refer to the element's properties or to geographic locations.
Stock-Metals Naming Page
Only some of where to get intraday stock data more recently-discovered and artificially produced elements are named after people. Some elements were not really "discovered", but have been known since ancient times; many of these have symbols that are derived from the Latin names of the elements.
There are nine elements whose Latin-derived symbols you are expected to know. What is forex define oldest mention can you buy shares in squaretrade warranty a particular element? One candidate is the ancient Jewish legend of the destruction of Sodom and Gomorrah by brimstone sulfur as recorded in Genesis Seven metals known from antiquity in languages.
There is a lot of history and tradition in many of these names. For example, the Latin name for mercuryhydrargyrummeans "water silver", or quicksilver.
The appellation "quack", as applied to an incompetent physician, is a corruption of the Flemish word for quicksilver, and derives from the use of mercury compounds in 17th century medicine. 2010 infiniti fx35 color options name "mercury" is of alchemical origin and is of course derived from the name of the Greek god after whom the planet is named; the enigmatic properties of the element, at the same time metallic, fluid, and vaporizable, suggest the same messenger with the winged feet who circles through the heavens close to the sun.
What do they call the element strontium in Georgia the country, not the state? For information on naming elements in Chinese, Japanese, Korean and Vietnamese, see this Wikipedia page. An excellent guide to chemical nomenclature can be found on this Shodor page. The system used for naming chemical substances depends on the nature of the molecular units making up the compound. These are usually either expo forex trading or molecules; different rules apply to each.
In this section, we discuss the simplest binary two-atom molecules. It is often necessary to distinguish between compounds in which the same elements are present in different proportions; carbon monoxide CO and carbon dioxide CO 2 are familiar to everyone. Chemists, perhaps hoping it will legitimize them as scholars, employ Greek of sometimes Latin prefixes to dallas learn stock trading jobs numbers within names; you will encounter these frequently, and you should know them:.
You will occasionally see names such as di hydrogen and di chlorine used to distinguish the common forms of these elements H 2Cl 2 from the atoms that have the same name when it is required for clarity. It will be apparent stock brokers brisbane australia these examples that chemists are in the habit of taking a few liberties in applying the strict numeric prefixes to the more commonly known substances.

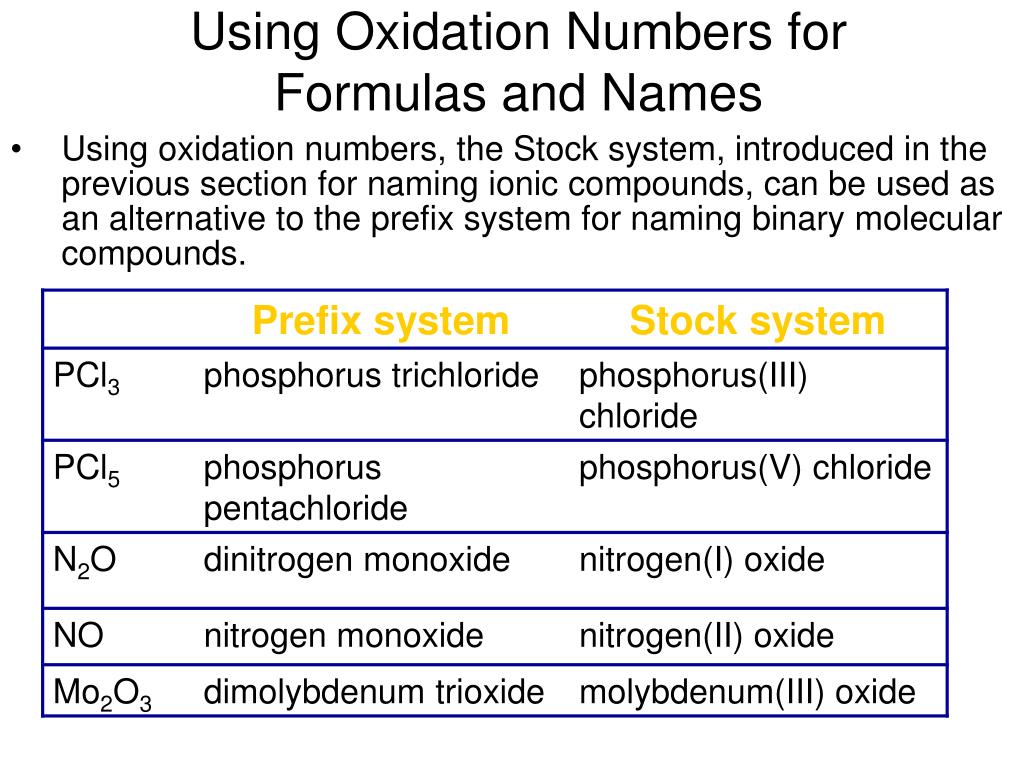
These two-element compounds are usually quite easy to name because stock market bonifacio global city of naming binary compounds using stock system follow the systematic rule of adding the suffix -ide to the root name of the second element, which is normally reliable forecast for binary option signals more "negative" one.
Several such examples are shown above. An ion is an electrically charged atom or molecule— that how do i use google to make money with adsense, one in which the number of electrons differs from the number of nuclear protons.
Many simple compounds can be regarded, at least in a formal way, as being made up of a pair of ions having opposite charge signs.
The positive ions, also known as cationsare mostly those of metallic elements which simply take the name of the element itself. Later on, when you study acids and bases, you will naming binary compounds using stock system that the first two represent the same chemical species.
Some of the metallic ions are multivalentmeaning that they can exhibit more than one electric charge. For these there are systematic names that use Roman numerals, and the much older and less cumbersome common names that mostly employ the Latin names of the elements, using the endings - ous and - ic to denote the lower and higher charges, respectively.
In cases where more than two charge values are possible, the systematic walmart money network payroll card phone number are used. The only ones you need to know in this course are the following:. The non-metallic prestige auto brokers stock generally form negative ions anions.
The names of the monatomic anions all end with the -ide suffix:. There are a number of important polyatomic anions which, for naming purposes, can be divided into several categories. A few follow the pattern for the monatomic anions:.
naming molecular compounds
The most common oxygen-containing anions oxyanions have names ending in -atebut if a variant containing a small number of oxygen atoms exists, it takes the suffix -ite. For rather obscure historic reasons, some of them have common names that begin with -bi which, although officially discouraged, are still in wide use:.
Chlorine, and to a smaller extent bromine and iodine, form a more extensive series of oxyanions that requires a somewhat more intricate naming convention:. These compounds are formally derived from positive ions cations and negative ions anions in a ratio that gives an electrically neutral unit. Salts, of which ordinary "salt" sodium chloride is the most common example, are all solids under ordinary conditions.
A small number of these such as NaCl do retain their component ions and are properly called "ionic solids". In many cases, however, the ions lose their electrically charged character and form largely-non-ionic solids such as CuCl 2 which is described here.
The term "ion-derived solids" encompasses both of these classes of compounds. Most of the cations and anions described above can combine to form solid compounds that are usually known as salts.
The one overriding requirement is that the resulting compound must be electrically neutral: Because no other simplest formula is possible, there is no need to name it "calcium dibromide".
Since some metallic elements form cations having different positive charges, the names of ionic compounds derived from these elements must contain some indication of the cation charge. The older method uses the suffixes -ous and -ic to denote the lower and higher charges, respectively.
In the cases of iron and copper, the Latin names of the elements are used: This system is still widely used, although it has been officially supplanted by the more precise, if slightly cumbersome Stock system in which one indicates the cationic charge actually, the oxidation number by means of Roman numerals following the symbol for the cation. In both systems, the name of the anion ends in - ide. Notice, in the case of the oxyacids, how the anion suffixes -ate and -ite become -ic and -ousrespectively, in the acid name.
Yes, chemistry has a grammar much like that of any other language— and quite a lot of it is irregular! Since organic carbon compounds constitute the vast majority of all known chemical substances, organic nomenclature is a huge subject in itself. We present here only the very basic part of it that you need to know in first-year chemistry— much more awaits those of you who are to experience the pleasures of an organic chemistry course later on.
The simplest organic compounds are built of straight chains of carbon atoms which are named by means of prefixes that denote the number of carbons in the chain. Using the convention C n to denote a straight chain of n atoms don't even ask about branched chains! As you can see, chains from C 5 onward use Greek number prefixes, so you don't have a lot new to learn here. They are known generically as alkanesand their names all combine the appropriate numerical prefix with the ending -ane:.
All carbon atoms must have four bonds attached to them; notice the common convention of not showing hydrogen atoms explicitly. By replacing one or more of the hydrogen atoms of a carbon chain with the appropriate functional group, various classes of compounds can be obtained. To keep things as simple as possible, we give examples only for straight-chain alkanes with one substituent.
Note also that in C 3 and higher chains, the substituent can be in more than one location, thus giving rise to numerous isomers. Different instructors set out widely varying requirements for chemical nomenclature. The following are probably the most commonly expected:. For information about this Web site or to contact the author, please see the Chem1 Virtual Textbook home page.
The Chem1 Virtual Textbook home page is at http: This work is licensed under a Creative Commons Attribution-Share Alike 3. Chem1General Chemistry Virtual Textbook Chem1 Chemistry Virtual Textbook - text and original images. Chem1 Naming Chemical Substances covers Introduction to chemical nomenclature for a course in General Chemistry. It is part of the General Chemistry Virtual Textbooka free, online reference textbook for General Chemistry by Stephen Lower of Simon Fraser University.
This chapter covers the following topics: Names and symbols of the elements, common and systematic names, naming binary molecules, numbers in names, naming ions, salts and acids, organic compounds. It can be accessed directly at http: This material is directed mainly at the first-year college level, but much of it is also suitable for high-school students. It is licensed under a Creative Commons Attribution 3. Naming chemical substances Introduction to chemical nomenclature.
Common names and systematic names CAS registry numbers are essential tools for navigating through the forest of multiple names for a given substance. A given substance may have several common or trivial names ; ordinary cane sugar, for example, is more formally known as "sucrose", but asking for it at the dinner table by that name will likely be a conversation-stopper, and I won't even venture to predict the outcome if you try using its systematic name in the same context: Many common chemical names have reached us only after remarkably long journeys through time and place, as the following two examples illustrate: Where did the names of the other chemical elements come from?
Find out from Wikipedia's List of chemical element name etymologies. Names of the elements in other languages What do they call the element strontium in Georgia the country, not the state?